PCBs, or polychlorinated biphenyls, are a group of oily, man-made chemicals that were used as fire preventives and insulators in electrical equipment such as transformers and capacitors until the government banned the manufacture of PCBs in 1977. (PCBs had many other uses as well.*) They were formerly called dimethyl chlorides and also went by the trade names “Aroclor” and “Halowax.” PCBs are often described as colorless, although they can be yellowish in color. They are also often described as odorless, although people who worked with them can identify PCBs by what they describe as a very distinctive odor.
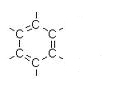
| Chemically, PCBs consist of two carbon rings. Chemists call each carbon ring a phenyl group. Each letter C represents a carbon atom. | |
| Usually chemists draw the ring without labeling the carbon atoms. It’s understood that there is a carbon atom at each corner. The six arms that stick out to the sides are places where other atoms can attach. | |
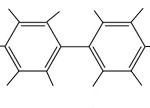
| If we put two of these phenyl groups together, we have a biphenyl group. (the prefix bi means two.) | |
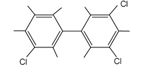
| If a biphenyl group contains a chlorine atom, it’s called a chlorinated biphenyl group. (Chlorinated means that it has chlorine atoms attached.) If the biphenyl group has multiple chlorine atoms attached, it is called a polychlorinated biphenyl. (The prefix poly means many or multiple.). PCBs consist of two carbon rings with attached chlorine atoms. The number of attached chlorines on a PCB molecule can be anywhere from 1 to 10. So this is how PCBs were named. PCB stands for polychlorinated biphenyl. |
PCBs are stable molecules. They do not break down or decompose naturally. In fact, this is one of the main reasons PCBs were so valuable to industry – they did not break down or decompose. Attempts at breaking down PCBs by using extremely high temperatures often result in the production of equally dangerous dioxins.
Since 1977, there has been a ban on the manufacturing of PCBs in the United States. Despite this ban, PCBs still persist in the environment, and they are allowed to remain in some pre-existing products in which the PCBs are totally contained.
*The Connecticut Department of Environmental Protection provides the following list of PCB uses: adhesives, asphalt roofing materials, carbonless copy paper, caulking, compressor oil, de-dusting agents, dielectric fluid, dyes, electromagnets, fluorescent light ballasts, grout, heat transfer fluid, inks, insulating coatings, lubricants, natural gas pipeline, paints, pesticides, plasticizers, rubberizers, space heaters, submersible well pumps, tar paper, wax extenders.